TOP NEWS, LATEST NEWS, BREAKING NEWS, HELP TIPS, SPORTS NEWS, WORLD NEWS, CELEBRITY NEWS. POPULAR NEWS, LIFE STYLE, FASHION, GENERAL NEWS.
Showing posts with label medicine. Show all posts
Showing posts with label medicine. Show all posts
Sunday, May 6, 2012
Wednesday, October 12, 2011
Vitamins use health & death risk in for women
Research & Study: Vitamins use may increase health & death risk in older women
Popping vitamins may do more harm than good, according to a new study that adds to a growing body of evidence suggesting some supplements may have health risks.
Older women who took a daily vitamin supplement, even just a multivitamin, had an increased risk of dying of cardiovascular disease and cancer, according to a study published Monday in the journal Archives of Internal Medicine.
Multivitamin users older women just as likely as non-users to have died or been diagnosed with common cancers or suffered stroke or a heart attack.
This study about woman who take supplements dying younger a good example of why it’s important to be research literate. This study does not show a casual link to taking supplements and early death.
In this study, being trumpeted all over the web today, WebMB reports that “In a new study, multivitamins, folic acid, iron, copper, magnesium, zinc, and vitamin B6 supplements all increased an older woman’s risk. The greatest risk was seen with iron supplements. Calcium supplements, however, seemed to reduce a woman’s risk of dying.
Researchers used data from the Iowa Women's Health Study to examine the link between vitamin and mineral supplements and death rates among 38,772 women, average age 61.6. Women filled out questionnaires about supplement use in 1986, 1997 and 2004. "Out of 15 studied supplements, seven are associated with increased total mortality risk," . "Other studies have not shown the mortality risk our study shows” lead author Jaakko Murso says, at the University of Minnesota in Minneapolis.
The new study linked a number of individual vitamins and minerals to the slight mortality risk, including multivitamins, vitamin B6, folic acid, iron, magnesium, zinc and copper.
"However, we do know that most compounds are toxic in high amounts, and long-term use might predispose [a person] to detrimental outcomes," he told MyHealthNewsDaily
Researchers used data from the Iowa Women's Health Study to examine the association between vitamin and mineral supplements and death rate among 38,772 women, average age 61.6 years.
"Out of 15 studied supplements, seven are associated with increased total mortality risk," says Murso. Among findings:
Use of multivitamins, vitamin B6, folic acid, iron, magnesium, zinc and copper are associated with increased risk of death.
The association between supplement intake and death risk was strongest with iron.
Calcium supplements, meanwhile, were associated with reduced risk.
Labels:
cancer,
cardiovascular,
death,
disease,
HEALTH,
increase,
medicine,
Multivitamin,
Research,
risk,
Study,
use,
Vitamins,
women
Tuesday, September 20, 2011
Surgeons Separate Conjoined Twin Girls who’s Heads
Surgeons Separate Conjoined Twin Girls who’s Heads :Rital And Ritag Gaboura
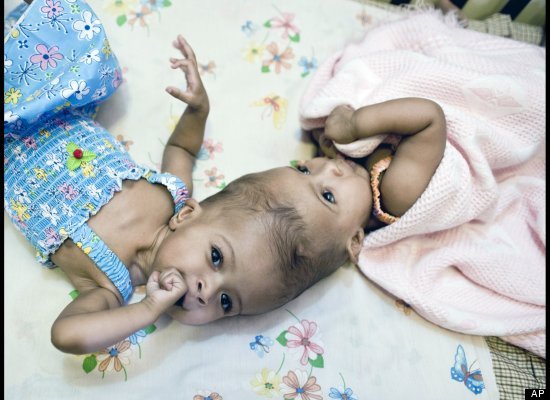
Sudanese twins born with the tops of their heads joined together have been separated in a rare and risky series of operations at a London children's hospital, officials said Sunday.
Facing the World, a charity which helps disfigured children, said it had helped fund the four-stage operation on 11-month-olds Rital and Ritag Gaboura.
Twins born joined at the head are known as craniopagus twins and they occur in about one in 2.5 million births. Separating them can be dangerous, especially if — as in this case — there's significant blood flow between their brains.
"It's extremely high-risk," said Dr. James Goodrich, who co-ordinated a similar separation of conjoined twins at New York's Montefiore Children's Hospital in 2004.
But the alternative can be just as bad. Because conjoined twins almost never pump the blood across their bodies evenly, the strongest sibling strains his or her heart trying to pick up the slack. Facing the World said that Ritag's overworked heart was already failing by the time her family arrived in Britain.
The separation took place in stages at London's Great Ormond Street Hospital. Two operations took place in May. Tissue expanders — essentially balloons intended to help stretch the babies' skin over their newly exposed heads — were inserted in July. The final separation took place on Aug. 15.
"Incidences of surviving twins with this condition is extremely rare," lead surgeon David Dunaway said in a statement released by the charity. "The task presented innumerable challenges and we were all very aware of our responsibilities to the family and these two little girls."
The after photograph showed both side-by-side looking alert and healthy, clutching white stuffed animals.
"Within days the twins were back on the general ward interacting and playing as before," the charity said. Its executive co-ordinator, Sarah Driver-Jowitt, predicted that the girls' parents — who haven't been named — may soon return home "with two healthy, separate girls."
Facing the World, a charity which helps disfigured children, said it had helped fund the four-stage operation on 11-month-olds Rital and Ritag Gaboura.
Twins born joined at the head are known as craniopagus twins and they occur in about one in 2.5 million births. Separating them can be dangerous, especially if — as in this case — there's significant blood flow between their brains.
"It's extremely high-risk," said Dr. James Goodrich, who co-ordinated a similar separation of conjoined twins at New York's Montefiore Children's Hospital in 2004.
But the alternative can be just as bad. Because conjoined twins almost never pump the blood across their bodies evenly, the strongest sibling strains his or her heart trying to pick up the slack. Facing the World said that Ritag's overworked heart was already failing by the time her family arrived in Britain.
The separation took place in stages at London's Great Ormond Street Hospital. Two operations took place in May. Tissue expanders — essentially balloons intended to help stretch the babies' skin over their newly exposed heads — were inserted in July. The final separation took place on Aug. 15.
"Incidences of surviving twins with this condition is extremely rare," lead surgeon David Dunaway said in a statement released by the charity. "The task presented innumerable challenges and we were all very aware of our responsibilities to the family and these two little girls."
The after photograph showed both side-by-side looking alert and healthy, clutching white stuffed animals.
"Within days the twins were back on the general ward interacting and playing as before," the charity said. Its executive co-ordinator, Sarah Driver-Jowitt, predicted that the girls' parents — who haven't been named — may soon return home "with two healthy, separate girls."
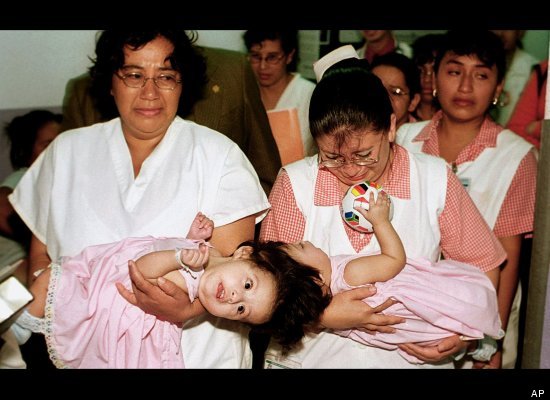
Although rare, operations to separate twins linked by their heads aren't unheard of. The U.S. National Library of Medicine records that one of the first successful operations to separate craniopagus twins took place in 1956.
Thursday, August 25, 2011
Allergan’s Botox Cleared by U.S.
Allergan’s Botox Cleared by U.S. FDA for Urinary Incontinence; Shares Gain
Allergan Inc. Won U.S. clearance to market the wrinkle smoother Botox as a treatment for urinary incontinence.
The Food and Drug Administration approved the drug for people with overactive bladders who have neurologic conditions such as multiple sclerosis and spinal cord injuries, the agency said today in a statement.
The medicine is injected into the bladder to increase its capacity by relaxing muscles.
Botox, also used to reduce facial wrinkles and treat neurological disorders, is the Irvine, California-based company’s top product with $1.4 billion in revenue last year. Sales of the drug for incontinence in people with MS and spinal cord injuries may reach $40 million in 2017, Seamus Fernandez, a Boston-based analyst at Leerink Swann & Co., said today in a note to investors.
“This approval of Botox is an important milestone in Allergan’s commitment to develop and make available novel treatment options for urologists and their patients,” Scott Whitcup, Allergan’s chief scientific officer and executive vice president for research and development, said running in a statement.
May bode well for eventual FDA clearance of Botox for idiopathic overactive bladder, a more common condition that may boost the drug’s sales by $210 million in 2017, Fernandez said. He said he expects Allergan to seek FDA clearance for that use next year, he said.
Botox, a purified form of the poison botulinum that blocks connections to nerves, won FDA approval in October as a treatment for chronic migraine headaches.
To contact the editor responsible for this story: Adriel Bettelheim at abettelheim@bloomberg.net
The Food and Drug Administration approved the drug for people with overactive bladders who have neurologic conditions such as multiple sclerosis and spinal cord injuries, the agency said today in a statement.
The medicine is injected into the bladder to increase its capacity by relaxing muscles.
Botox, also used to reduce facial wrinkles and treat neurological disorders, is the Irvine, California-based company’s top product with $1.4 billion in revenue last year. Sales of the drug for incontinence in people with MS and spinal cord injuries may reach $40 million in 2017, Seamus Fernandez, a Boston-based analyst at Leerink Swann & Co., said today in a note to investors.
“This approval of Botox is an important milestone in Allergan’s commitment to develop and make available novel treatment options for urologists and their patients,” Scott Whitcup, Allergan’s chief scientific officer and executive vice president for research and development, said running in a statement.
May bode well for eventual FDA clearance of Botox for idiopathic overactive bladder, a more common condition that may boost the drug’s sales by $210 million in 2017, Fernandez said. He said he expects Allergan to seek FDA clearance for that use next year, he said.
Botox, a purified form of the poison botulinum that blocks connections to nerves, won FDA approval in October as a treatment for chronic migraine headaches.
To contact the editor responsible for this story: Adriel Bettelheim at abettelheim@bloomberg.net
or
http://helptipslatestnews.blogspot.com/
http://helptipslatestnews.blogspot.com/
or
http://secure.signup-page.com/3884/17117/COX
http://secure.signup-page.com/3884/17117/COX
source of http://www.bloomberg.com/news/2011-08-24/allergan-s-botox-cleared-by-u-s-fda-for-urinary-incontinence-shares-gain.html
Labels:
Allergans,
Botox,
Cleared,
Drug,
FDA,
food,
headaches,
Incontinence,
medicine,
multiple,
sclerosisinjuries,
Urinary,
US
Subscribe to:
Posts (Atom)


